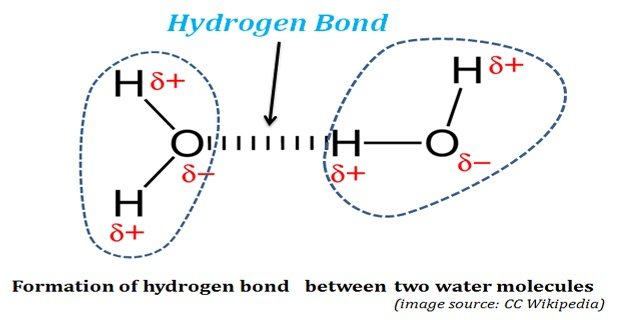
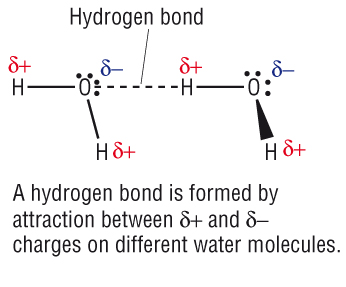
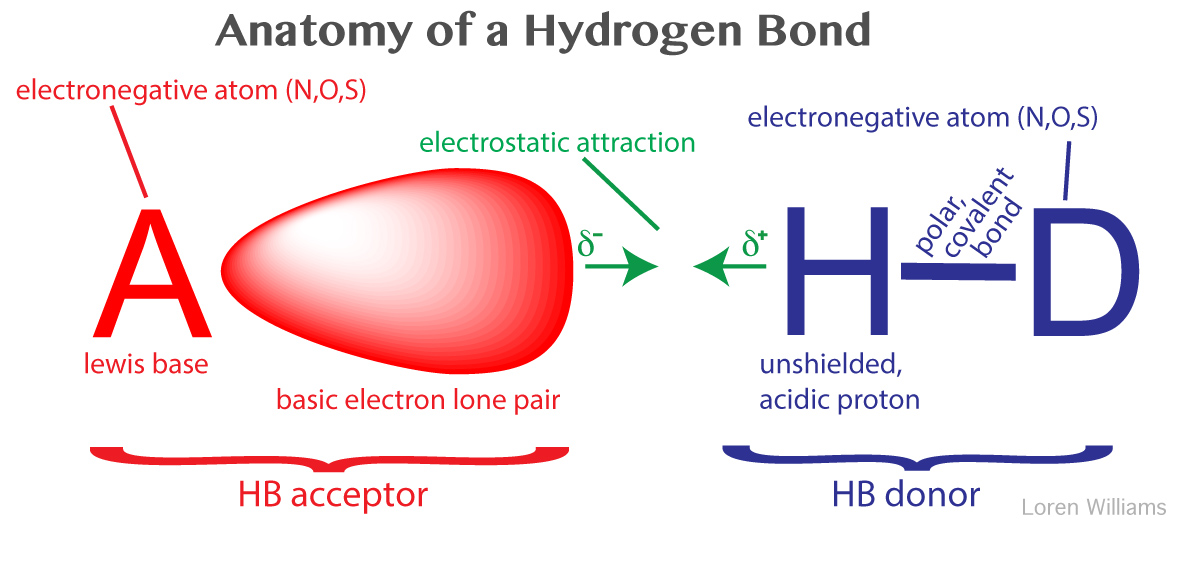
Hidrogen Bond

It results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a n o or f atom and another very.
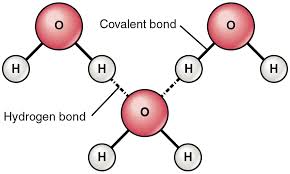
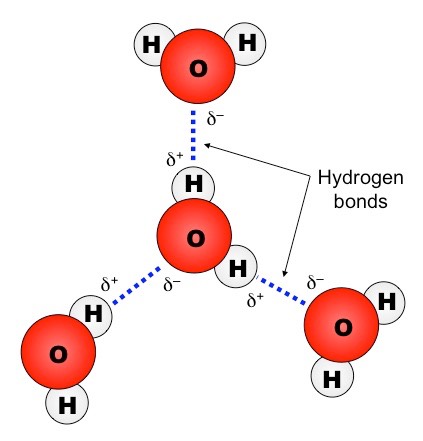
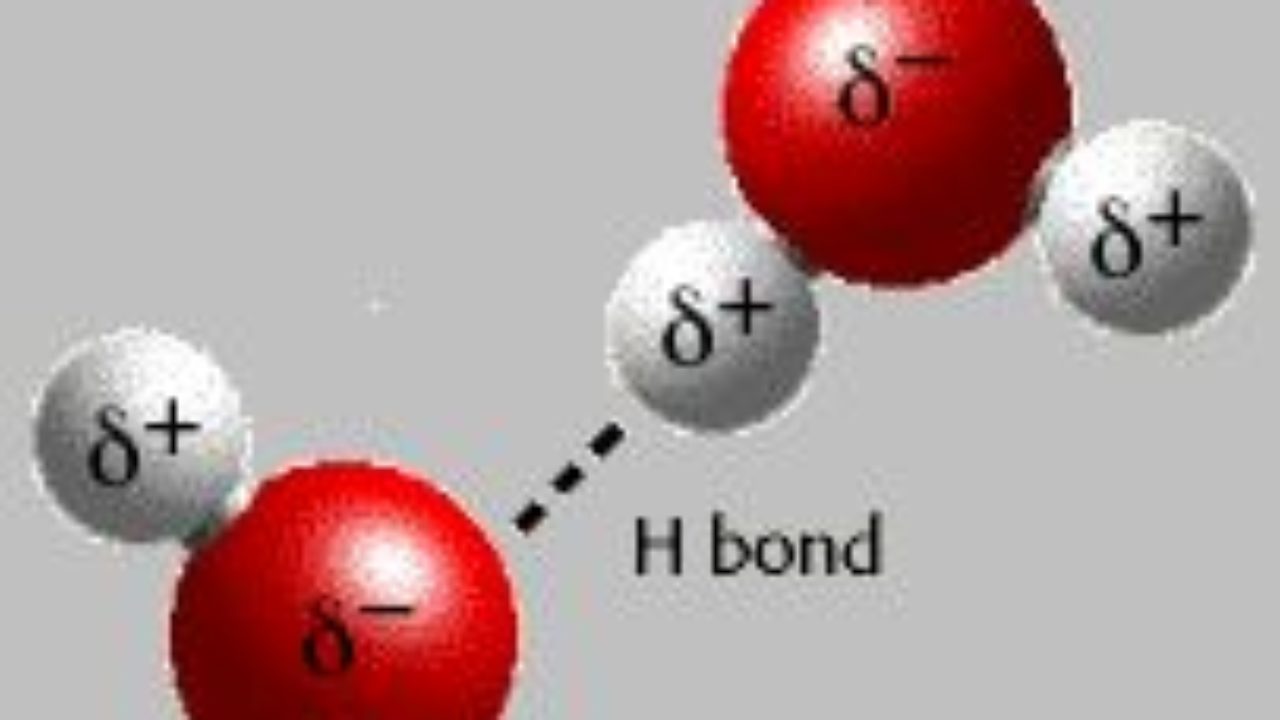
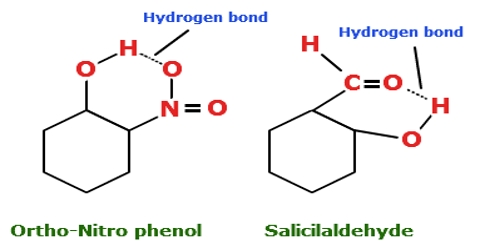
Hidrogen bond. Sometimes the bonding is intramolecular or between atoms of a molecule rather than between atoms of separate molecules intermolecular. Examples of hydrogen bonds. Hydrogen bonds may form between atoms within a molecule or between two separate molecules. The plus end of one a hydrogen atom associates with the minus end of another an oxygen atom.
Carbon hydrogen bonds have a bond length of about 1 09 å 1 09 10 10 m and a bond. Hydrogen bonding interaction involving a hydrogen atom located between a pair of other atoms having a high affinity for electrons. Hydrogen bonding is a special type of dipole dipole attraction between molecules not a covalent bond to a hydrogen atom. This completes both of their outer shells making them stable.
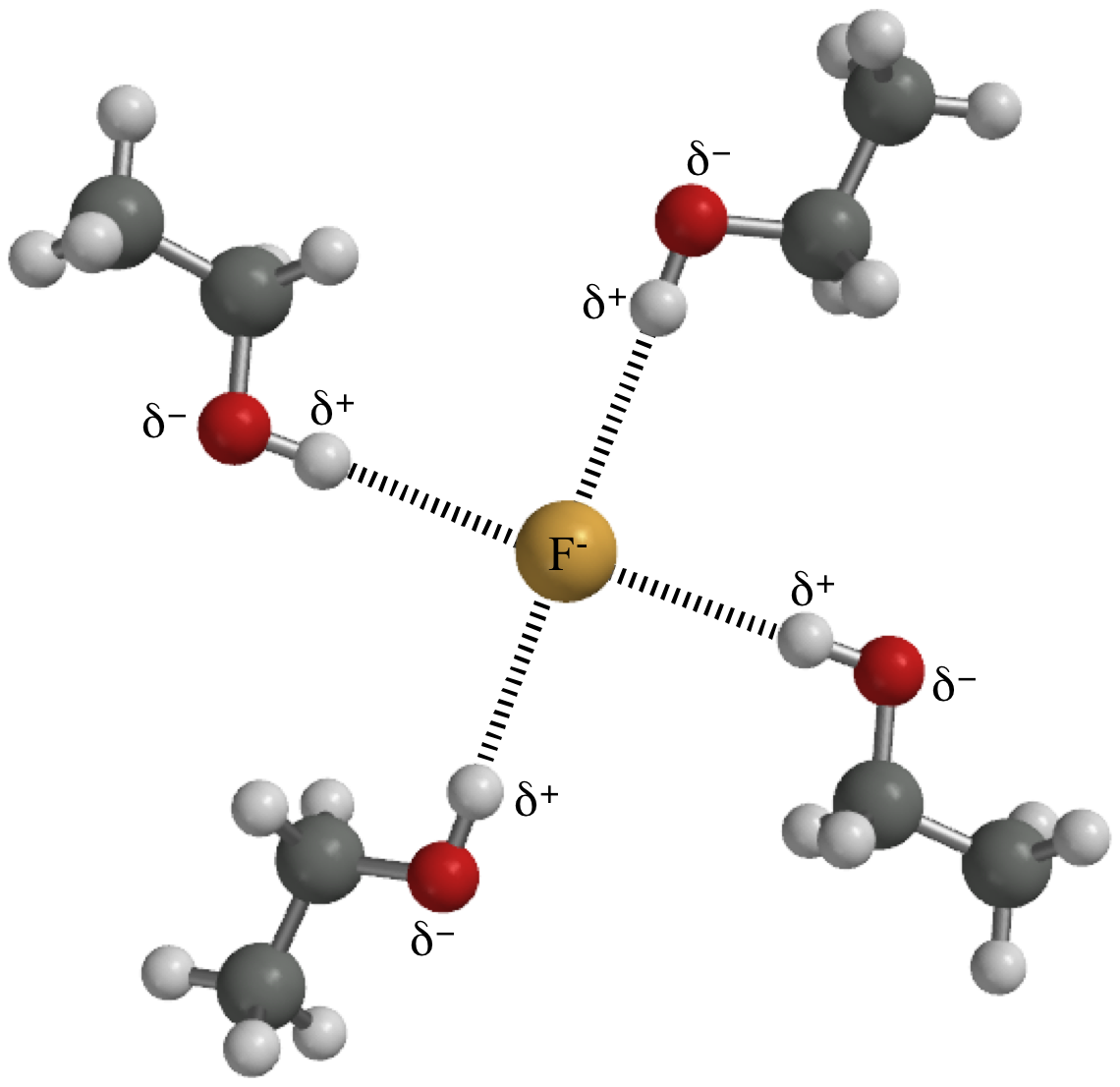
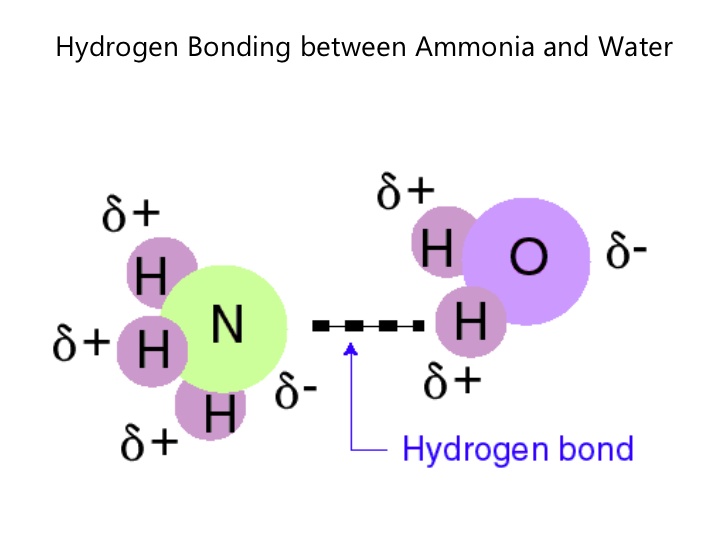
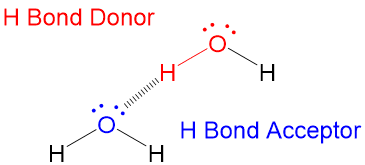
A hydrogen bond is an attraction between two atoms that already participate in other chemical bonds. A hydrogen bond be both intermolecular and intramolecular. A hydrogen bond often informally abbreviated h bond is a partial intermolecular bonding interaction between a lone pair on an electron rich donor atom particularly the second row elements nitrogen n oxygen o or fluorine f and the antibonding molecular orbital of a bond between hydrogen h and a more electronegative atom or group. Usually hydrogen bonds occur between hydrogenand fluorine oxygen or nitrogen.
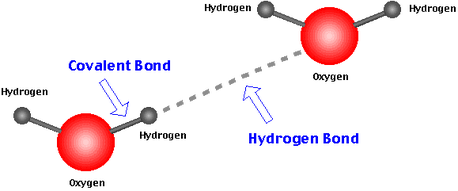
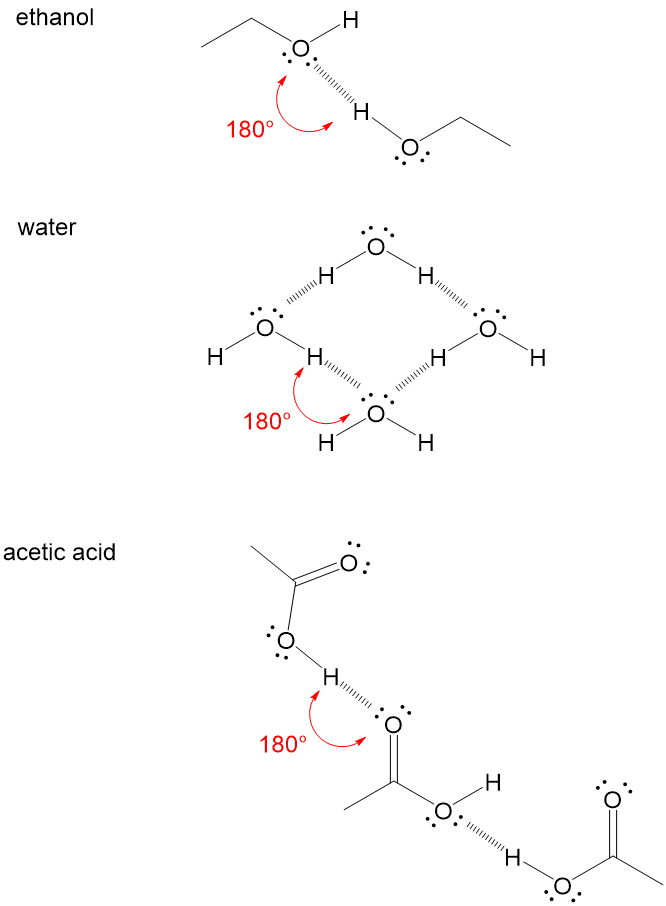
Hydrogen bond a chemical bond formed between an electropositive atom typically hydrogen and a strongly electronegative atom such as oxygen or nitrogen. Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces. This bond is a covalent bond meaning that carbon shares its outer valence electrons with up to four hydrogens. Hydrogen bonds are responsible for the bonding of water molecules in liquid and solid states and are weaker than covalent and ionic bonds.
One of the atoms is hydrogen while the other may be any electronegative atom such as oxygen chlorine or fluorine. Hydrogen bonds can exist between atoms in different molecules or in parts of the same molecule. These attractions are an example of hydrogen bonds weak interactions that form between a hydrogen with a partial positive charge and a more electronegative atom such as oxygen. A hydrogen bond is a dipole dipole interaction between a hydrogen bound to a highly electronegative atom like nitrogen or oxygen and another atom with a partial negative charge.
An electrostatic attraction between a hydrogen atom in one polar molecule as of water and a small electronegative atom as of oxygen nitrogen or fluorine in usually another molecule of the same or a different polar substance other words from hydrogen bond example sentences learn more about hydrogen bond.
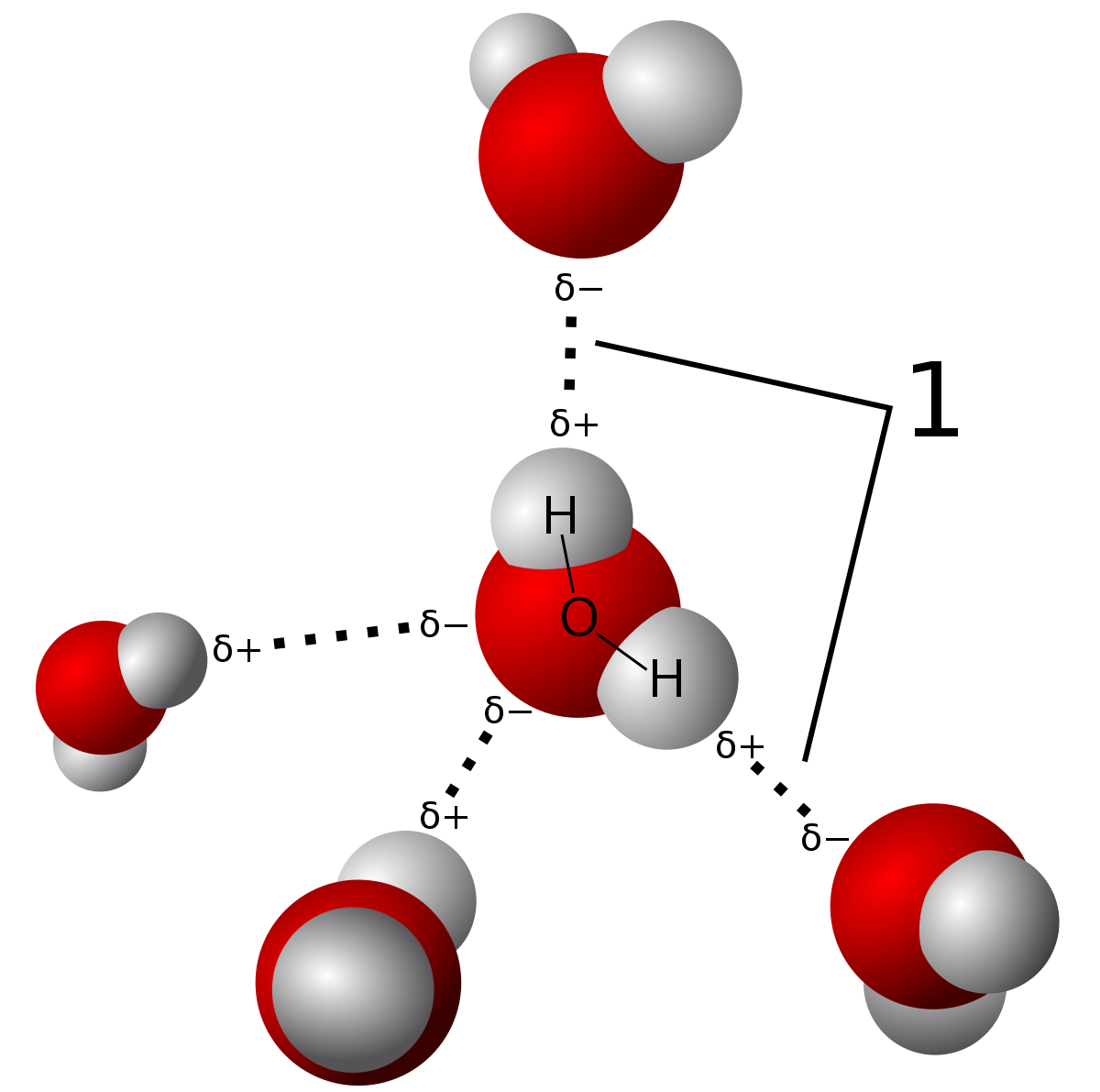


















:max_bytes(150000):strip_icc()/water-molecules-136810077-5c448a6ec9e77c0001568fb2.jpg)