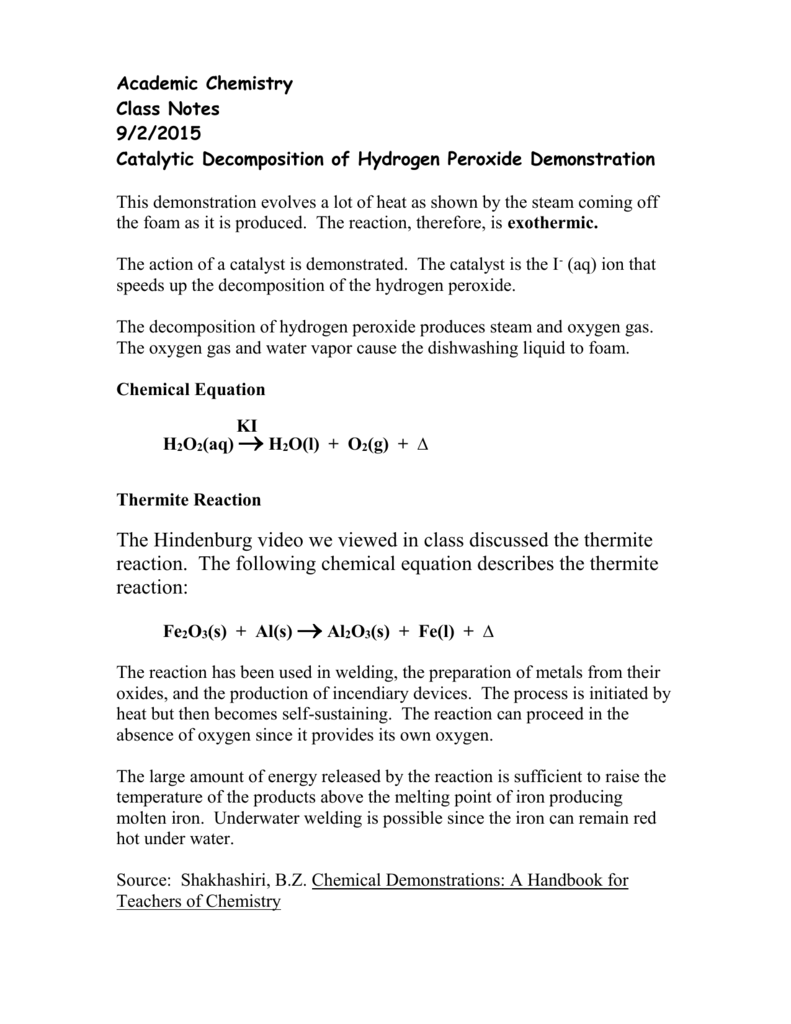
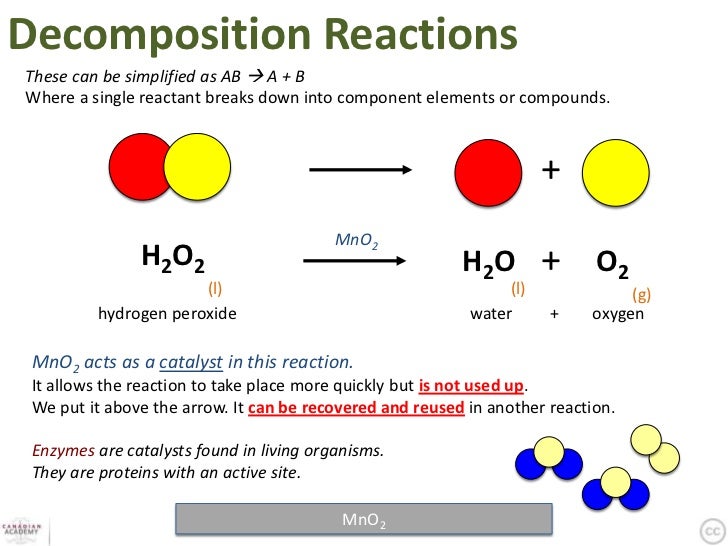
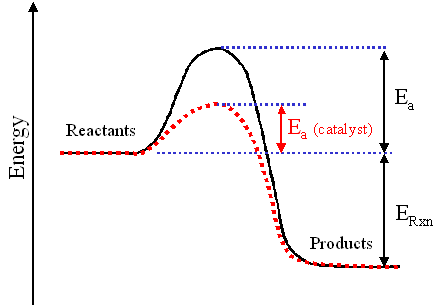
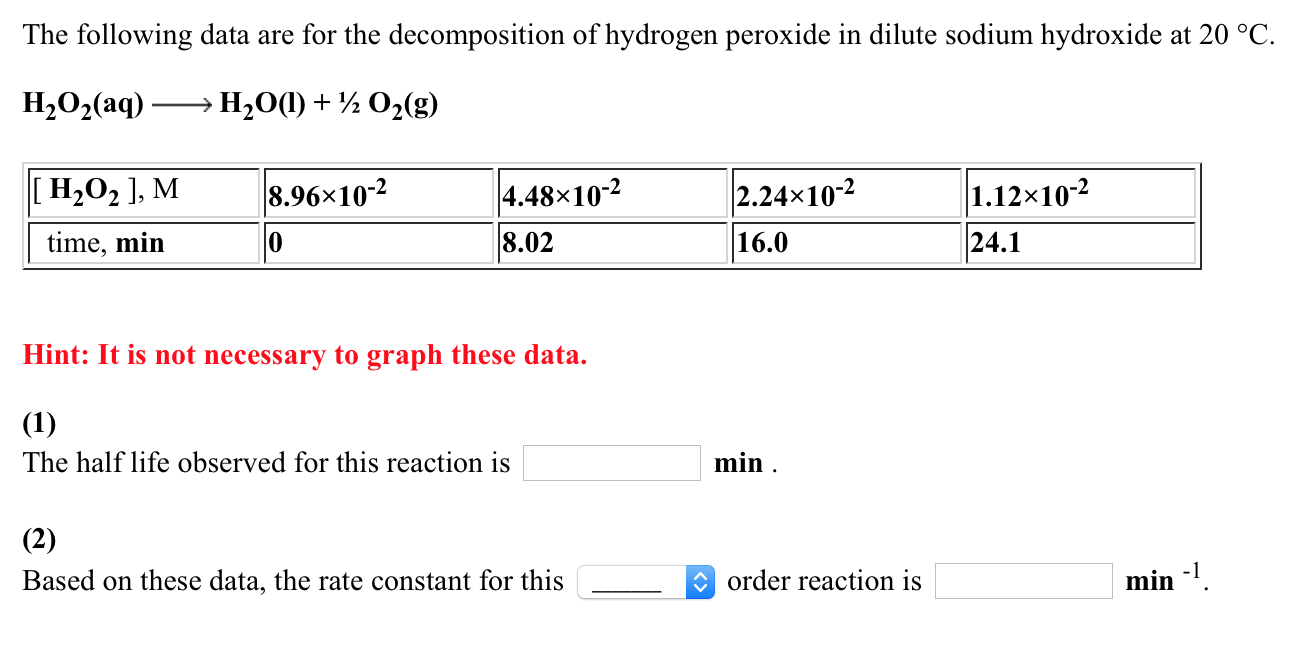
H2o2 Decomposition Reaction

Hydrogen peroxide can also be decomposed biologically by the enzyme catalase.
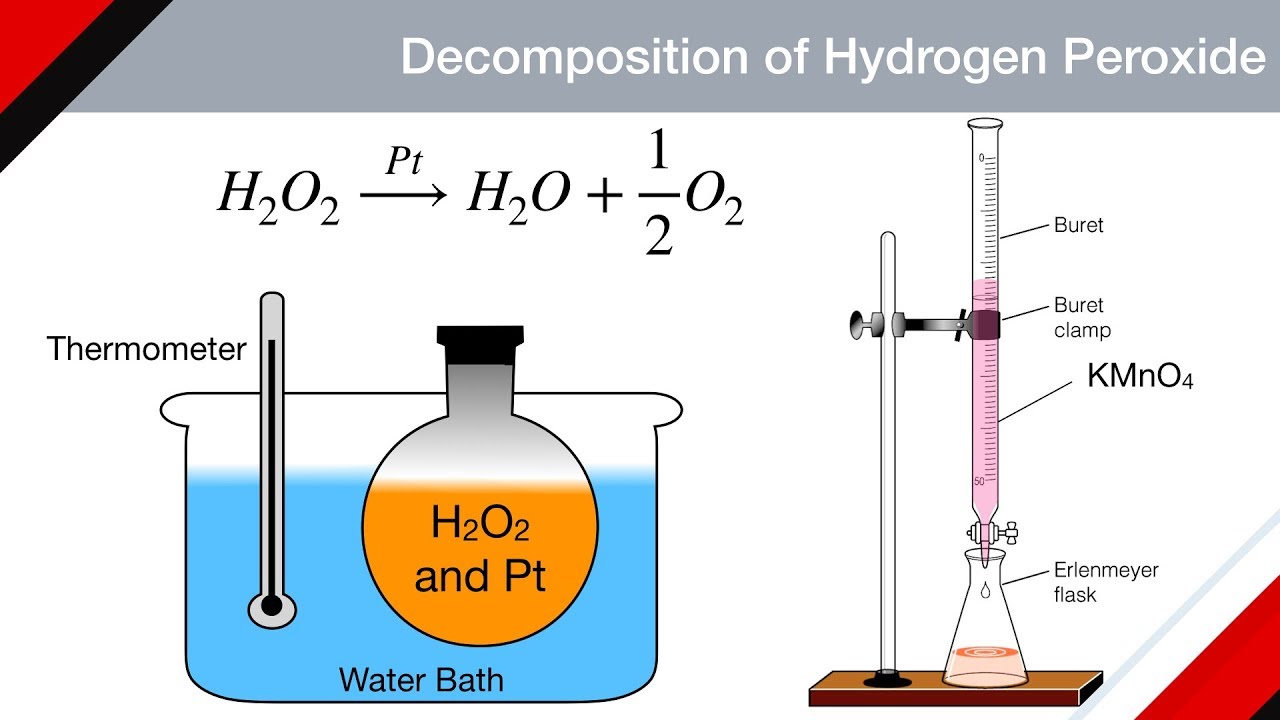
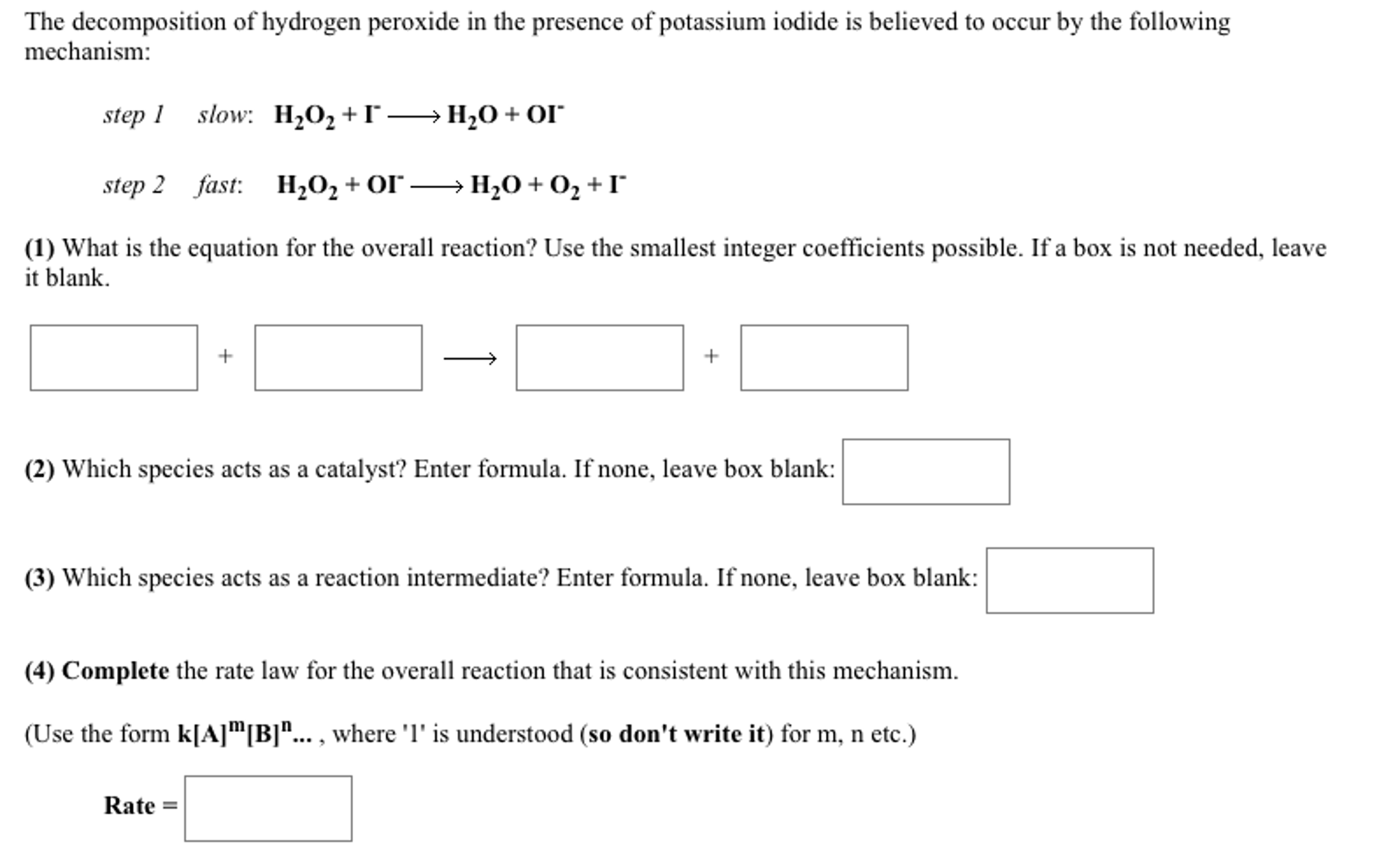
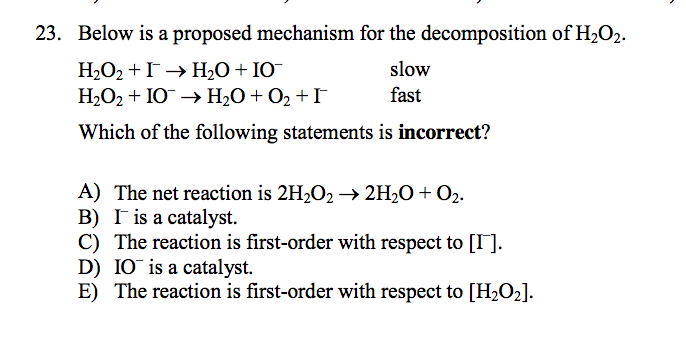
H2o2 decomposition reaction. The standard free energy change δ f o is 27 92 kcal mole at 25 c rapid decomposition of concentrated h2o2 solutions may not be complete with concentrations up to 10 remaining. In the presence of potassium iodide mno 2 aqueous naoh will also catalyze the decomposition of h 2 o 2. Secondly there are two reaction in the process actually one is reaction between kmno4 and h2o2 which non catalytic decomposition. The redox properties of hydrogen peroxide depend on ph.
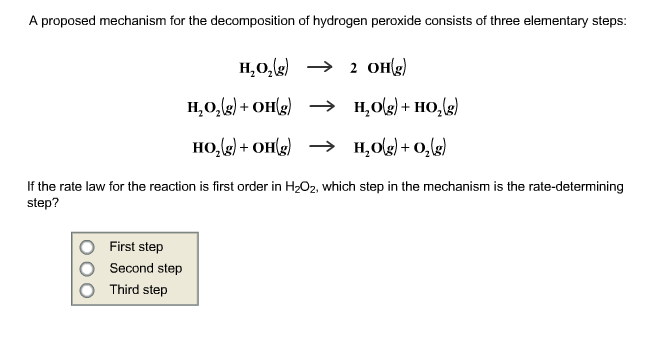
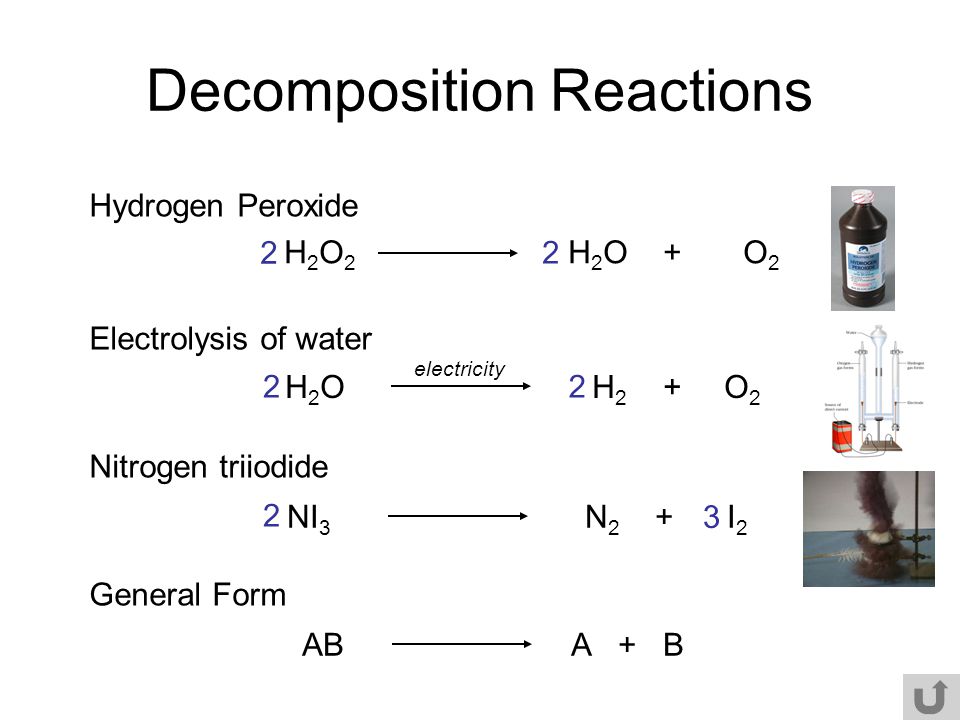
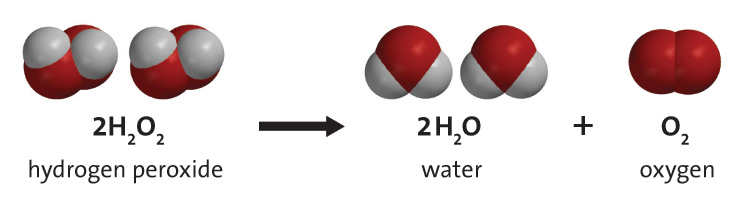
2 h2o2 aq 2 h2o o2 g. The decomposition takes place according to the reaction below. Click here to get an answer to your question decomposition of h2o2 follows a first order reaction. Hydrogen peroxide is injurious to cells because it attacks unsaturated fatty acids lipids found in cell membranes and consequently cells produce a powerful catalyst catalase that decomposes h2o2 keusch.
Under higher temperatures and concentrations it decomposes to form water and oxygen. Even 10 h2o2 can boil if it becomes grossly contaminated. Decomposition of hydrogen peroxide can be catalysed by other compounds such as transition metals like silver and platinum. In fifty minutes the concentration of h2o2 decreases from 0 5 to 0 125 m in one such decomposition.
The effect of temperature is such that an increase of 10 c increases the rate of decomposition by a factor of 2 3 i e a first order rate equation. Firstly hydrogen peroxide is partly oxdized to oxygen and the other part is reduced to water it is so called a disproportionation reaction. This reaction can be catalyzed by minute traces of transition metal ions. Decomposition of hydrogen peroxide hydrogen peroxide decomposes into water and oxygen gas o 2 when temperature increases or exposing to the sunlight.
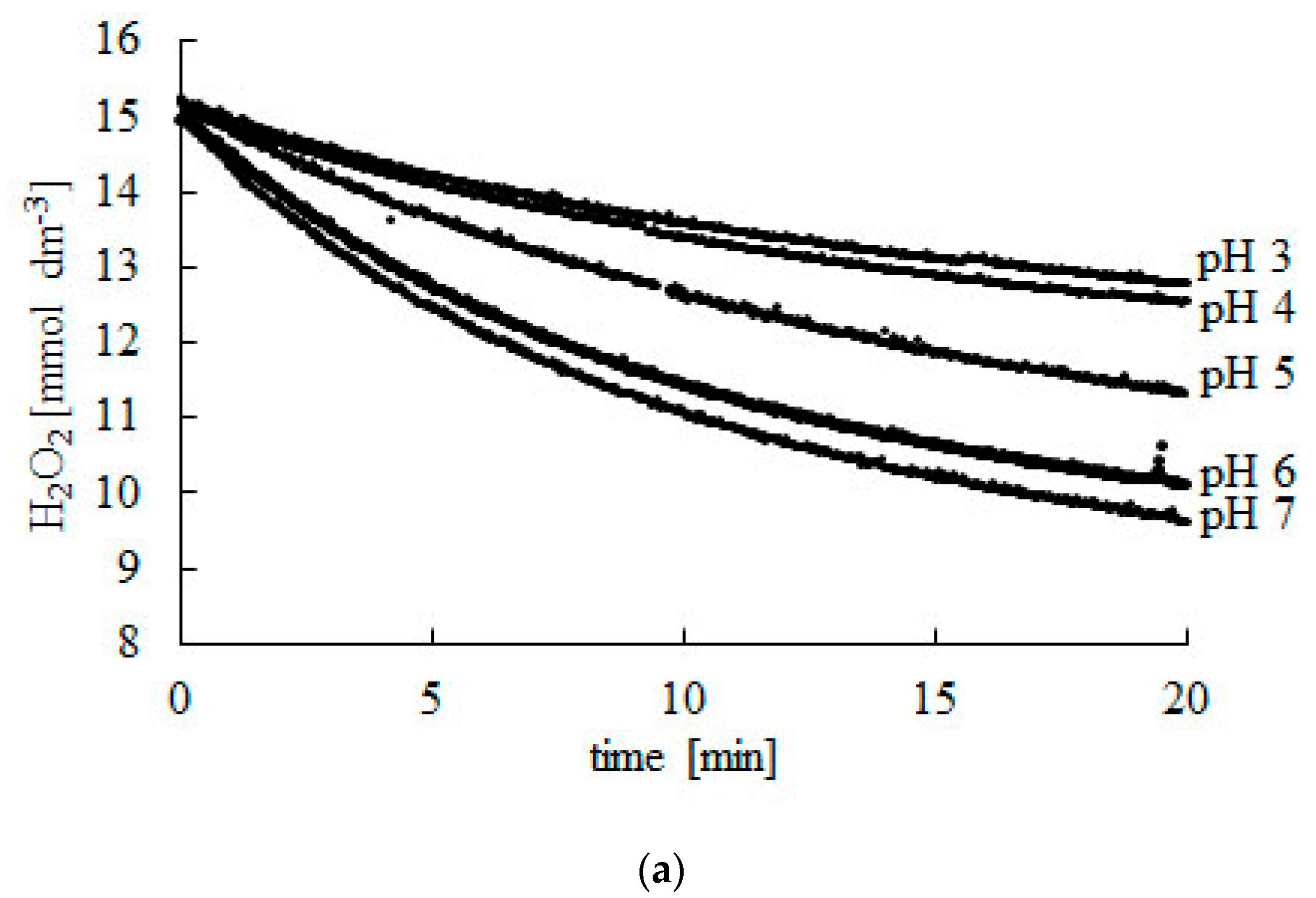
Of hydrogen peroxide the decomposition of hydrogen peroxide in aqueous solution proceeds very slowly. Depending on the ph level hydrogen peroxide has powerful reducing and oxidising redox properties. Catalase is a catalyst rather than just another reactant because it reacts both as an electron donor and as an electron acceptor. The decomposition of hydrogen peroxide liberates oxygen and heat.
H2o2 decomposition is highly exothermic 23 44 kcal mole. In this study we demonstrate that the addition of ascorbate can lower the conversion percentage of h2o2 to o2 from 71 to 11 and thus increase the.